Regenerative Medicine of Cardiovascular Diseases
(Deutsch: Lehrstuhl für Regenerative Medizin kardiovaskulärer Erkrankungen)
Recent advances in developmental and stem cell biology have paved the way for a wide spectrum of novel cardiovascular therapies, from the transplantation of stem cell-derived cells to the stimulation of endogenous regeneration processes. However, there are still many basic scientific questions to answer before these concepts can be successfully translated into patient treatment. Addressing such questions using state-of-the-art methods is the overarching goal of our group.
In the research projects outlined below, we combine 2D and 3D in vitro models based on human pluripotent stem cells with animal models such as the mouse and pig. Another important facet of our work is the use of CRISPR/Cas9 gene editing for many applications including the generation of reporter cell lines and the design of novel gene therapy approaches.
The heart is the first organ to form and has a complex structure consisting of many specialized cell types populating the heart muscle, blood vessels, and connective tissue. During early cardiac development, cells must multiply rapidly but also specify, i.e. limit their developmental potential and determine into which cell type they will later differentiate. Our group investigates how different cardiovascular progenitor populations arise, specify, and differentiate, notably by dissecting lineage trajectories via single-cell transcriptomics and chromatin accessibility profiling. We are particularly interested in how these processes are disrupted in congenital heart defects and how we can use knowledge from development to develop new regenerative approaches in adults.
One of our areas of interest is the role of transcription factors such as Isl1, which during heart development is expressed in so-called second heart field (SHF) cardiovascular progenitors that later mainly contribute to the right ventricle, the outflow tract and portions of the atria.
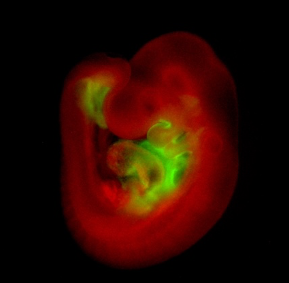
Whole-mount immunohistological staining of an Isl1Cre/+;R26mTmG/+ embryo at embryonic day 9.5 showing Isl1-Cre mediated mG (green) marking of SHF progenitors and their derivatives.
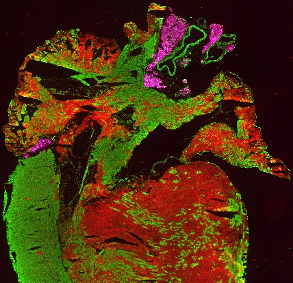
Immunohistological staining of a heart section from an Isl1Cre/+;R26mTmG/+ mouse at 4 weeks, showing Isl1-Cre mediated mG (green) marking of the right ventricle, parts of the left ventricle and both atria. Perilipin1 (magenta) marks adipocytes.
Human induced pluripotent stem cells (hiPSCs) are stem cells that are derived from the somatic cells (e.g. blood or skin cells) of healthy individuals or patients. They have the capacity to self-renew indefinitely and to give rise to any cell type of the body in vitro, making them an exceptionally powerful tool for studying human disease in patient-specific backgrounds. A major focus of our research is using hiPSC-based disease modelling to gain insights into the molecular and cellular mechanisms involved in cardiovascular disorders such as arrhythmogenic right ventricular cardiomyopathy (ARVC), hypoplastic left heart syndrome (HLHS), cardiomyopathy associated with Noonan syndrome, left ventricular non-compaction (LVNC), and others. Once a therapeutic target has been identified, we also use hiPSC-derived cells as a platform for testing novel therapies, as we have previously done for Duchenne muscular dystrophy (DMD).
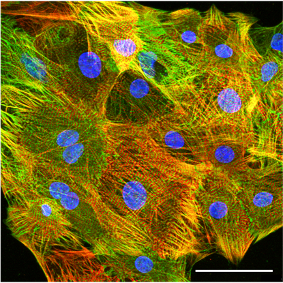
Confocal immunofluorescence image of hiPSC-derived cardiomyocytes stained for the sarcomere proteins cardiac troponin T (red) and a-actinin (green). Scale bar: 50 µm.

Live immunofluorescence imaging of a 3D cardiac spheroid derived from a double reporter stem cell line expressing eGFP and mCherry in the endogenous loci of the cardiac transcription factors NKX2.5 and TBX5, respectively. Scale bar: 200 µm.
As cells cultured in 2D fail to recapitulate certain features of their in vivo counterparts, part of our work is dedicated to developing 3D cardiac models that better mimic the physiology of heart tissue. We routinely produce engineered heart tissue based on hiPSC-derived cardiac cells seeded on decellularized ventricular matrix from pigs or non-human primates, which we cultivate in a biomimetic chamber system promoting tissue maturation by continuous mechanical and electrical stimulation. We have also established an ex vivo embryo culture system that allows us to study the function of human cardiac cells in human-mouse or human-pig chimeras. Finally, we recently generated human pluripotent stem cell-derived cardiac organoids showing morphological, functional, and molecular self-organization of ventricular myocardium and epicardium.
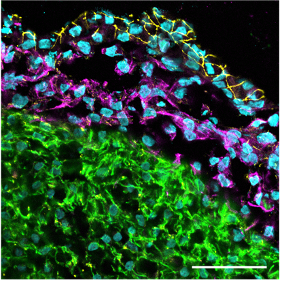
Confocal immunofluorescence image of a cardiac organoid section showing the outer epicardial layer (tight junction protein 1 staining in yellow), the subjacent epicardium-derived cells (EPDCs) having undergone epithelial-to-mesenchymal transition (vimentin staining in magenta) and the myocardial layer (cardiac troponin T staining in green). Scale bar: 50 µm.
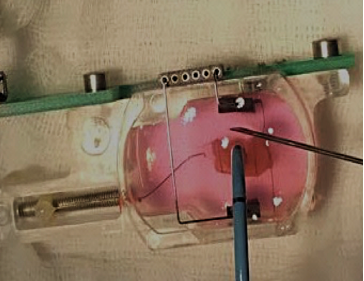
Biomimetic chamber containing engineered heart tissue undergoing radiofrequency ablation to mimic an acute myocardial injury.
- Generation, culture and differentiation of human pluripotent stem cells in 2D and 3D
- CRISPR/Cas9 gene editing for the generation of engineered reporter lines and knock-in/knock-out lines
- Functional characterization of cardiomyocytes via calcium imaging, optical action potential recordings, contractility measurements
- Bulk and single-cell RNA-sequencing and ATAC-sequencing
- Immunofluorescence analysis, confocal microscopy
- Flow cytometry and FACS sorting
- Cell injection in embryos and ex vivo embryo culture
- Molecular biology techniques including cloning, digital PCR
- Capillary Western Blot, chromatin immunoprecipitation
-
Prof. Dr. Alessandra Moretti
Chair of Regenerative Medicine of Cardiovascular Diseases, group leader -
Dr. Tatjana Dorn
Senior scientist (developmental biology, lineage decision) -
Dr. Monika Nowak-Imialek
Veterinarian scientist (porcine expanded pluripotent stem cells & human-pig chimeras) -
Dr. Gianluca Santamaria
Postdoc (bioinformatic data analysis) -
Dr. Alexander Göedel
Physician scientist (bioinformatic data analysis) -
Dr. Christine Schneider
Physician scientist (3D tissue engineering & functional imaging) -
Sophie Zengerle
PhD student (organoids and modeling of congenital heart disease) -
Sinem Sürmeli
PhD student (CRISPR-based genome editing) -
Luis Felipe Monge Mora
PhD student (cardiac organiods and imaging) -
Marco Crovella
Technician (animals and histology) -
Christina Scherb
Technician (molecular biology) -
Birgit Campbell
Technician (cell culture and iPSC generation) -
Aylin Mayer
Technician (heart tissue slices andd gene editing)
Scientific Projects:
We regularly offer projects for diploma, PhD and M.D. students.
Third-Party Funding:
- European Research Council - EPICURE
- German Research Foundation
- German Centre for Cardiovascular Research, Munich Heart
Alliance
- Else Kröner-Fresenius Foundation
Original work
Rawat H, Kornherr J, Zawada D, Bakhshiyeva S, Kupatt C, Laugwitz KL, Bähr A, Dorn T, Moretti A, Nowak-Imialek M. (2023) Recapitulating porcine cardiac development in vitro: from expanded potential stem cell to embryo culture models. Front Cell Dev Biol. 11:1111684. doi: 10.3389/fcell.2023.1111684. PMID: 37261075
Poch CM, Dendorfer A, Laugwitz KL, Moretti A. (2023) Ex vivo Culture and Contractile Force Measurements of Non-human Primate Heart Slices. Bio Protoc. 13(13):e4750. doi: 10.21769/BioProtoc.4750. PMID: 37456341
Meier AB, Zawada D, De Angelis MT, Martens LD, Santamaria G, Zengerle S, Nowak-Imialek M, Kornherr J, Zhang F, Tian Q, Wolf CM, Kupatt C, Sahara M, Lipp P, Theis FJ, Gagneur J, Goedel A, Laugwitz KL, Dorn T, Moretti A. (2023) Epicardioid single-cell genomics uncovers principles of human epicardium biology in heart development and disease. Nat Biotechnol. 41(12):1787-1800. doi: 10.1038/s41587-023-01718-7. PMID: 37012447
Zawada D, Kornherr J, Meier AB, Santamaria G, Dorn T, Nowak-Imialek M, Ortmann D, Zhang F, Lachmann M, Dreßen M, Ortiz M, Mascetti VL, Harmer SC, Nobles M, Tinker A, De Angelis MT, Pedersen RA, Grote P, Laugwitz KL, Moretti A, Goedel A. (2023) Retinoic acid signaling modulation guides in vitro specification of human heart field-specific progenitor pools. Nat Commun. 14(1):1722. doi: 10.1038/s41467-023-36764-x. PMID: 37012244
Poch CM, Foo KS, De Angelis MT, Jennbacken K, Santamaria G, Bähr A, Wang QD, Reiter F, Hornaschewitz N, Zawada D, Bozoglu T, My I, Meier A, Dorn T, Hege S, Lehtinen ML, Tsoi YL, Hovdal D, Hyllner J, Schwarz S, Sudhop S, Jurisch V, Sini M, Fellows MD, Cummings M, Clarke J, Baptista R, Eroglu E, Wolf E, Klymiuk N, Lu K, Tomasi R, Dendorfer A, Gaspari M, Parrotta E, Cuda G, Krane M, Sinnecker D, Hoppmann P, Kupatt C, Fritsche-Danielson R, Moretti A, Chien KR, Laugwitz KL. (2022) Migratory and anti-fibrotic programmes define the regenerative potential of human cardiac progenitors. Nat Cell Biol. 24(5):659-671. doi: 10.1038/s41556-022-00899-8. PMID: 35550611
Esfandyari D, Idrissou BMG, Hennis K, Avramopoulos P, Dueck A, El-Battrawy I, Grüter L, Meier MA, Näger AC, Ramanujam D, Dorn T, Meitinger T, Hagl C, Milting H, Borggrefe M, Fenske S, Biel M, Dendorfer A, Sassi Y, Moretti A, Engelhardt S. (2022) MicroRNA-365 regulates human cardiac action potential duration. Nat Commun. 13(1):220. doi: 10.1038/s41467-021-27856-7. PMID: 35017523
Krane M, Dreßen M, Santamaria G, My I, Schneider CM, Dorn T, Laue S, Mastantuono E, Berutti R, Rawat H, Gilsbach R, Schneider P, Lahm H, Schwarz S, Doppler SA, Paige S, Puluca N, Doll S, Neb I, Brade T, Zhang Z, Abou-Ajram C, Northoff B, Holdt LM, Sudhop S, Sahara M, Goedel A, Dendorfer A, Tjong FVY, Rijlaarsdam ME, Cleuziou J, Lang N, Kupatt C, Bezzina C, Lange R, Bowles NE, Mann M, Gelb BD, Crotti L, Hein L, Meitinger T, Wu S, Sinnecker D, Gruber PJ, Laugwitz KL, Moretti A. (2021) Sequential Defects in Cardiac Lineage Commitment and Maturation Cause Hypoplastic Left Heart Syndrome. Circulation. 144(17):1409-1428. doi: 10.1161/CIRCULATIONAHA.121.056198. PMID: 34694888
Moretti A, Fonteyne L, Giesert F, Hoppmann P, Meier AB, Bozoglu T, Baehr A, Schneider CM, Sinnecker D, Klett K, Fröhlich T, Rahman FA, Haufe T, Sun S, Jurisch V, Kessler B, Hinkel R, Dirschinger R, Martens E, Jilek C, Graf A, Krebs S, Santamaria G, Kurome M, Zakhartchenko V, Campbell B, Voelse K, Wolf A, Ziegler T, Reichert S, Lee S, Flenkenthaler F, Dorn T, Jeremias I, Blum H, Dendorfer A, Schnieke A, Krause S, Walter MC, Klymiuk N, Laugwitz KL, Wolf E, Wurst W, Kupatt C (2020). Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat Med 26(2):207-214
Guo Y, Dorn T, Kühl SJ, Linnemann A, Rothe M, Pfister AS, Vainio S, Laugwitz KL, Moretti A*, Kühl M* (2019). The Wnt inhibitor Dkk1 is required for maintaining the normal cardiac differentiation program in Xenopus laevis. Dev Biol. 449:1-13.
Dorn T, Kornherr J, Parrotta EI, Zawada D, Ayetey H, Santamaria G, Iop L, Mastantuono E, Sinnecker D, Goedel A, Dirschinger RJ, My I, Laue S, Bozoglu T, Baarlink C, Ziegler T, Graf E, Hinkel R, Cuda G, Kääb S, Grace AA, Grosse R, Kupatt C, Meitinger T, Smith AG, Laugwitz KL, Moretti A. (2018) Interplay of cell-cell contacts and RhoA/MRTF-A signaling regulates cardiomyocyte identity. EMBO J 37:pii: e98133.



